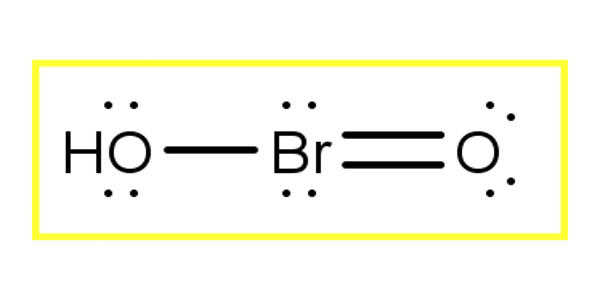
Bromous acid is the inorganic compound with the formula of HBrO2. It is an unstable compound, although salts of its conjugate base – bromites – have been isolated. In acidic solution, bromites decompose to bromine.
Discovery
In 1905, Richards A. H. proved the existence of bromous acid through a series of experiments involving silver nitrate (AgNO3) and bromine. The reaction of excess cold aqueous to form hypobromous acid (HBrO), silver bromide (AgBr) and nitric acid (HNO3):
- Br2 AgNO3 H2O → HBrO AgBr HNO3
Richards discovered that the effect of adding excess liquid bromine in a concentrated silver nitrate (AgNO3) resulted in a different reaction mechanism. From numbers of equivalent portions of acid bromine formed from the previous reaction, the ratio between oxygen and bromine was calculated, with the exact value of O:Br (0.149975:0.3745), suggesting the acid compound contains two oxygen atom to one bromine atom. Thus, the chemical structure of the acid compound was deducted as HBrO2.
According to Richards, hypobromous acid (HBrO) arises by the reaction of bromine and silver nitrate solution:
- Br2 AgNO3 H2O → HBrO AgBr HNO3
- 2 AgNO3 HBrO Br2 H2O → HBrO2 2 AgBr 2 HNO3
Isomerism
The molecule HBrO2 has a bent structure with ∠(H−O−Br) angles of 106.1°. HOBrO also adopts a non-planar conformation with one isomer structure (2a) adopting a dihedral angle ∠(H−O−Br−O) of 74.2°. Moreover, the planar structures of two other isomers (2b-cis and 2c-trans) are transition state for fast enantiomerization.
Another study identified three isomers: HOOBr, HOBrO, and HBr(O)O.
Synthesis
A oxidation reaction between hypobromous acid (HBrO) and hypochlorous acid (HClO) can be used to produce bromous acid (HBrO2) and hydrochloric acid (HCl).
- HBrO HClO → HBrO2 HCl
A redox reaction of hypobromous acid (HBrO) can form bromous acid (HBrO2) as its product:
- HBrO H2O − 2e− → HBrO2 2H
The disproportionation reaction of two equivalents hypobromous acid (HBrO) results in the formation of both bromous acid (HBrO2) and hydrobromic acid (HBr):
- 2 HBrO → HBrO2 HBr
A rearrangement reaction, which results from the syn-proportion of bromic acid (HBrO3) and hydrobromic acid (HBr) gives bromous acid (HBrO2):
- 2 HBrO3 HBr → 3 HBrO2
Salts
The salts NaBrO2·3H2O and Ba(BrO2)2·H2O have been crystallized. Upon treatment of these aqueous solutions with salts of Pb2 , Hg2 , and Ag , the corresponding heavy metal bromites precipitate as solids.
Belousov–Zhabotinsky reaction
Bromous acid is a product of the Belousov–Zhabotinsky reaction resulting from the combination of potassium bromate, cerium(IV) sulfate, propanedioic acid and citric acid in dilute sulfuric acid. Bromous acid is an intermediate stage of the reaction between bromate ion (BrO−
3 ) and bromine (Br−):
- BrO−
3 2 Br− → HBrO2 HBrO
Other relevant reactions in such oscillating reactions are:
- HBrO2 BrO−
3 H → 2 BrO•
2 H2O - 2 HBrO2 → BrO−
3 HOBr H
Bromites reduce permanganates to manganates (VI):
- 2 MnO−
4 BrO−
2 OH− → 2 MnO2−
4 BrO−
3 H2O
pKa measurement
The acid dissociation constant of bromous acid, Ka = [H ][BrO−
2]/[HBrO2], was determined using different methods.
The value of the pKa for bromous acid was estimated in research studying the decomposition of bromites. The research measured the rate of bromite decomposition as a function of hydrogen and bromite ion concentrations. The experimental data of the log of the initial velocity were plotted against pH. Using this method, the estimated pKa value for bromous acid was 6.25.
Using another method, the pKa for bromous acid was measured based on the initial velocity of the reaction between sodium bromites and potassium iodine in a pH range of 2.9–8.0, at 25 °C and ionic strength of 0.06 M. The first order dependence of the initial velocity of this disproportionation reaction on [H ] in a pH range of 4.5–8.0. The value of acid dissociation constant measured by this method is Ka = (3.7±0.9)×10−4 M and pKa = 3.43±0.05.
Reactivity
In comparison to other oxygen-centered oxidants (hypohalites, anions of peroxides) and in line with its low basicity, bromite is a rather weak nucleophile. Rate constants of bromite towards carbocations and acceptor-substituted olefins are by 1–3 orders of magnitude lower than the ones measured with hypobromite.
References
Further reading
- Ukeles, S.D.; Freiberg, M. (2002). "Bromine, Inorganic Compounds". Kirk-Othmer Encyclopedia of Chemical Technology. doi:10.1002/0471238961.0218151321110512.a01. ISBN 978-0-471-48494-3.